NIH grant fosters research into role 'R-loops' play in cancer formation
Jayme Blaschke | December 14, 2020

Xiaoyu Xue, assistant professor in the Department of Chemistry and Biochemistry at Texas State University, has received a three-year, $430,000 grant from the National Institute of General Medical Sciences of the National Institutes of Health to study how cells prevent R-loop accumulations to gain insight into possible avenues of cancer prevention.
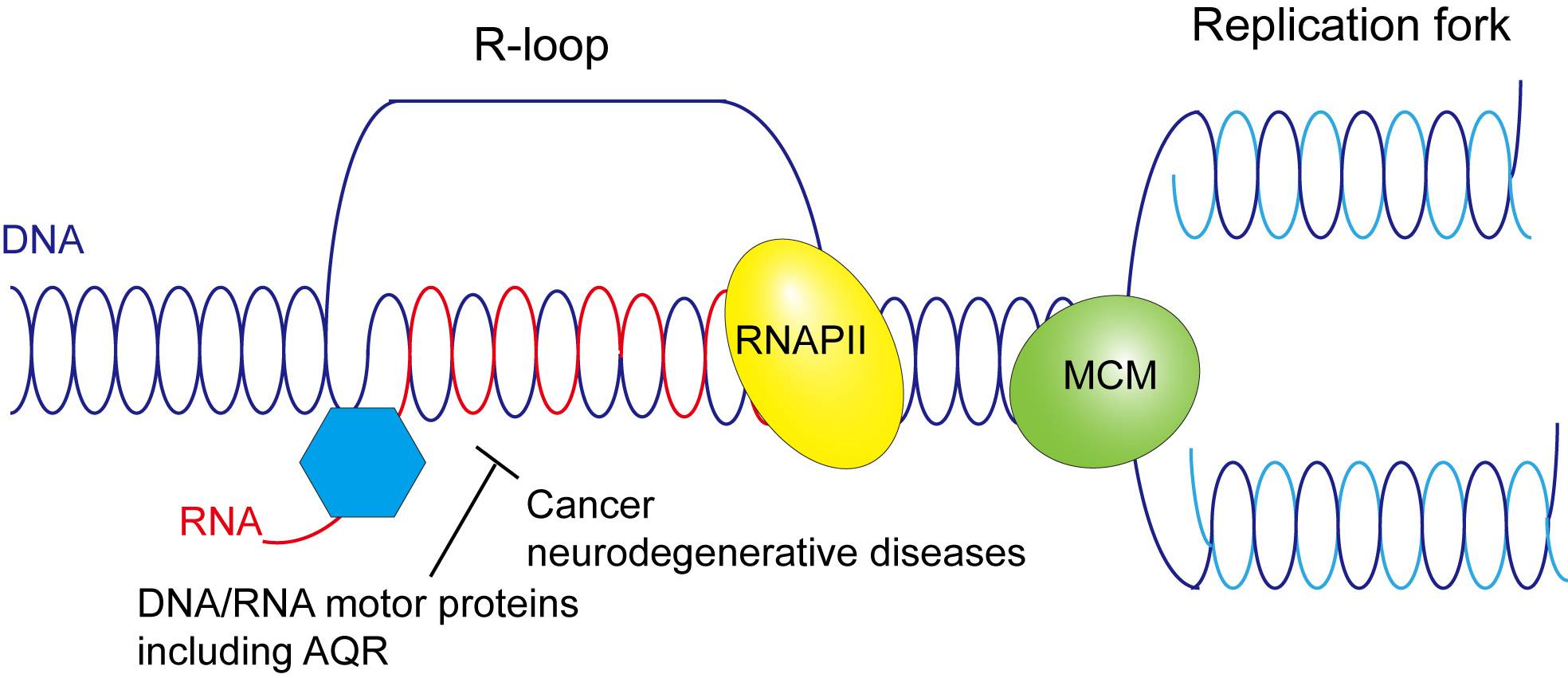
R-loops are three-stranded nucleic acid structures that contain an RNA strand being hybridized to the complementary strand of duplex DNA. They are induced by exposure to environmental stress that includes heavy metals, air pollutants and pesticides, as well as ultraviolet radiation. Importantly, R-loops can cause chromosome breakage and aberrations, leading to cell transformation and cancer.
"The results from our project will shed light on pathological R-loop removal as a way to avoid human diseases and maintain genome integrity," Xue said.
Cells have evolved different means to prevent R-loop accumulation. One relies on the use of DNA/RNA helicases, a type of protein that usually unwinds DNA and RNA duplexes into single stranded products. These helicases work to remove or dissociate pathological R-loops. The Texas State research focuses on identifying human helicases involved in R-loop dissociation and determining how they work at a molecular level.
The study builds upon Xue's previous research into helicase proteins such as AQR, known to play a crucial role in DNA replication and repair within cells. Data from Xue's earlier work indicates AQR resolves R-loops before they can reach harmful levels, so to better understand the causes of R-loop accumulation, Xue's research group is developing mutations of AQR incapable of R-loop resolution. By isolating DNA-binding or RNA-binding AQR mutants in the laboratory, Xue can determine if these mutations hamper the protein's normal function.
The NIH grant will allow Xue to expand the scope of his research to include UAP56, another human helicase that may also play a significant role in controlling R-loop accumulation.
The results from Xue's research will shed light on pathological R-loop removal as a way to avoid human diseases and maintain genome integrity. By identifying human helicases responsible for R-loop dissociation, the research will contribute to the development of novel strategies to treat cancer and neurodegenerative disease caused by R-loop accumulation.
Share this article
For more information, contact University Communications:Jayme Blaschke, 512-245-2555 Sandy Pantlik, 512-245-2922 |