Research into RNA-DNA 'R-loops' could shed light on cancer formation
Jayme Blaschke | August 13, 2019
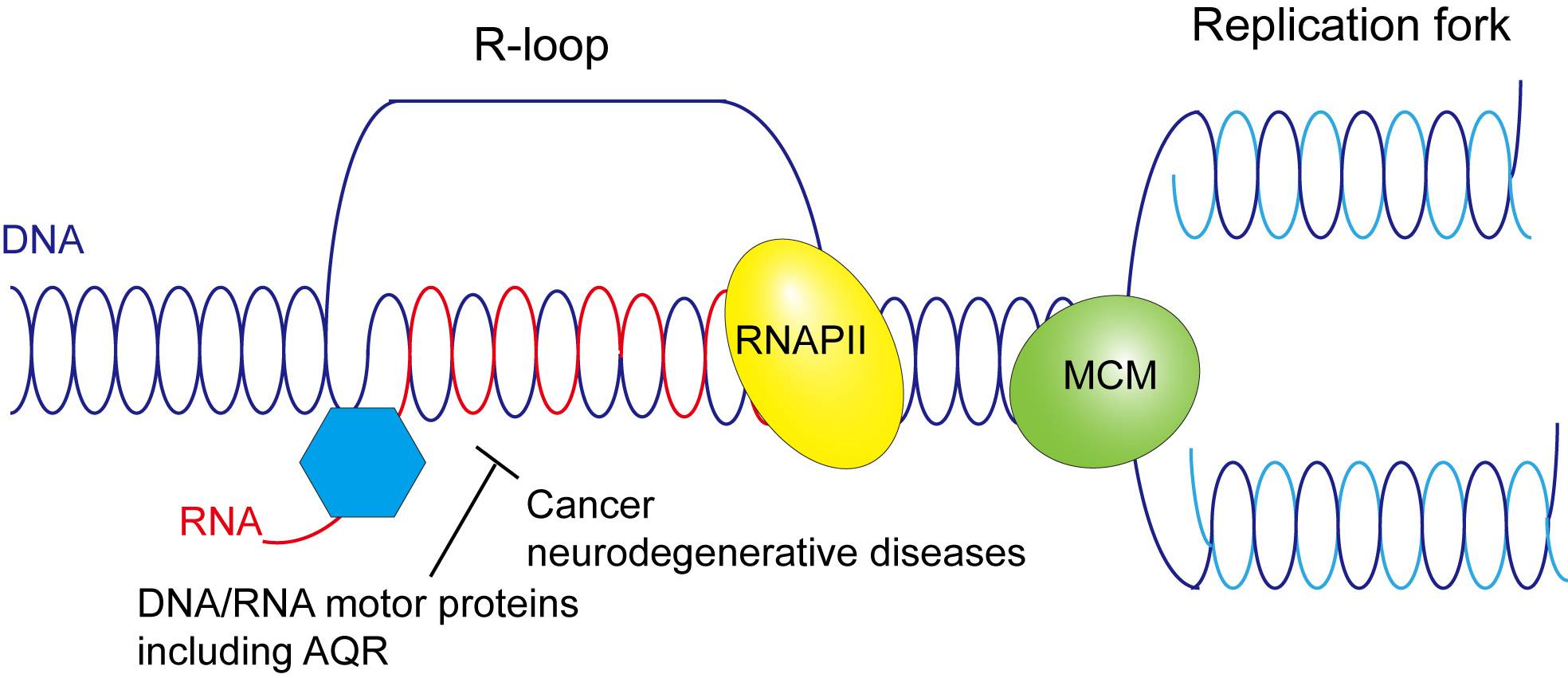
Accumulation of unusual RNA-DNA hybrids, known as R-loops, are often associated with cancers. Xiaoyu Xue, assistant professor in the Department of Chemistry and Biochemistry at Texas State University, is heading a research study examining the role of the human motor protein Aquarius (AQR) in resolving R-loops to gain insight into possible avenues of cancer prevention.
R-loops are a three-stranded nucleic acid structure induced by exposure to environmental stress that includes heavy metals, air pollutants and pesticides, as well as ultraviolet radiation. Xue is also looking into the relation between such oxidative stresses and R-loop accumulation in cells. The research is supported by a $424,000 grant from the National Institute of Environmental Health Sciences (NIEHS), which Xue received in 2018, prior to his arrival at Texas State from Yale University.
"Aberrant R-loop accumulation contributes to some neurodegenerative diseases and many cancers," Xue explained. "R-loop accumulations will cause replication stress and DNA double strand breaks, leading to genome instability and cancer formation."
Proteins such as AQR are known as helicases, and play a crucial role in DNA replication and repair within cells. Preliminary data from the study indicates AQR resolves R-loops before they can reach harmful levels, so to better understand the causes of R-loop accumulation, Xue's research group is developing mutations of AQR incapable of R-loop resolution. By isolating DNA-binding or RNA-binding AQR mutants in the laboratory, Xue can determine if these mutations hamper the protein's normal function.
"Properly functioning AQR dissociates pathological R-loops, which may reduce cancer risk. Some mutations of AQR may increase cancer risk," Xue said. "In fact, genome sequencing data reveals that 12 percent of melanoma harbor AQR mutations. We want to know if and how these AQR mutations affect AQR function in R-loop resolution."
Xue's group is studying these AQR mutants in vitro to determine what impact they have on R-loop accumulation and cancer formation. Simultaneously, Xue is collaborating with Andres Aguilera at the University of Seville and Cabimer in Spain, an expert in R-loop resolution and recombination, who is studying the same effects in cells to corroborate the results.
The next phase of the research will be identifying cofactors that regulate AQR function. Xue has already learned that RPA, a single-strand DNA binding protein, may serve a regulatory role over AQR. Xue is also looking to expand the scope of his research to include UAP56, another human helicase that may also play a role in controlling R-loop accumulation.
The research is shedding light on a poorly understood pathway of genome preservation and may contribute toward the development of novel strategies to avoid the accumulation of R-loops upon exposure to environmental stress. Ultimately, the findings may aid in the development of new treatments for neurodegenerative diseases and cancer.
Share this article
For more information, contact University Communications:Jayme Blaschke, 512-245-2555 Sandy Pantlik, 512-245-2922 |